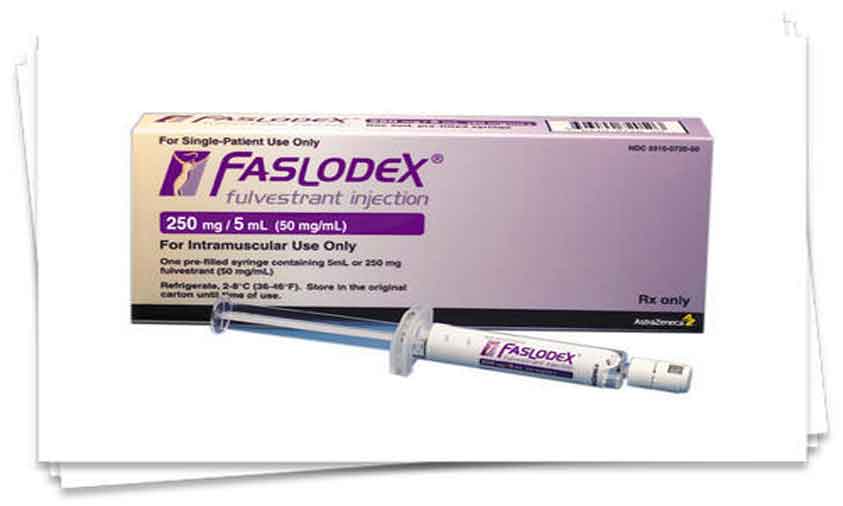
Hyderabad, Sept.7 (Hydnow): Dr. Reddy’s Laboratories Ltd. today announced the launch of Fulvestrant Injection, 250 mg/5 mL (50 mg/mL) per Single-dose Syringe, a therapeutic equivalent generic version of Faslodex (fulvestrant) Injection, 250 mg/5 mL (50 mg/mL), approved by the U.S. Food and Drug Administration (USFDA).
The Faslodex brand and generic market had U.S. sales of approximately $407 million MAT for the most recent twelve months ending in June 2020 according to IQVIA Health.
Dr. Reddy’s Fulvestrant Injection, 250 mg/5 mL (50 mg/mL) per Single-dose Syringe is available in a carton containing two 5 mL single-dose prefilled syringes. (Hydnow)
Advertisements